Medical device reports from between Oct. 15, 2018, and March 31, 2019, included three reports of death, 45 reports of patient infection and 159 reports of device contamination tied to inadequate reprocessing of duodenoscopes used in procedures involving the pancreas and bile duct, according to an article on the MedTech Dive website.
The Food and Drug Administration (FDA) also released interim results from post-market studies by device makers showing contamination rates in the scopes significantly higher than preliminary findings released in December.
A total of 5.4% of all samples tested positive for “high concern” bacteria, versus 3% reported previously.
Manufacturers Olympus, Fujifilm and Pentax have not complied with agreed-upon timetables for completing the post-market studies, FDA said.
 Regulations Take the Lead in Healthcare Restroom Design
Regulations Take the Lead in Healthcare Restroom Design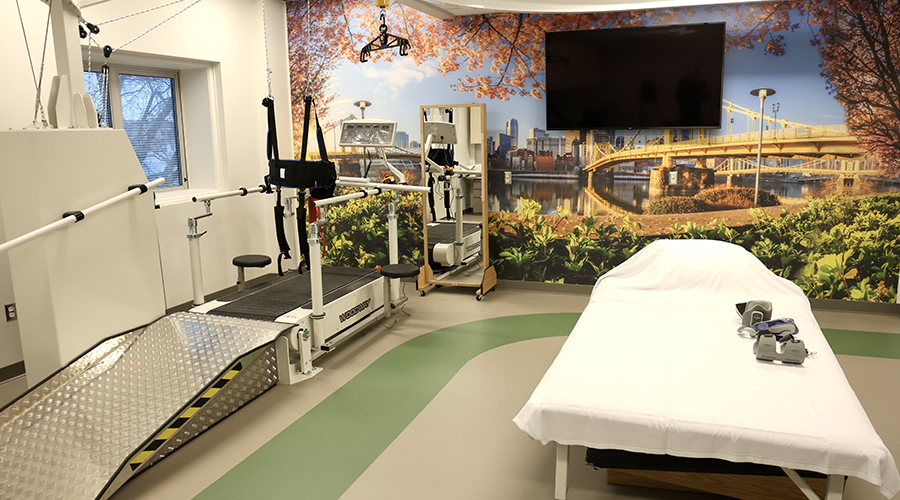 AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit
AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay
HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay Turning Facility Data Into ROI: Where Healthcare Leaders Should Start
Turning Facility Data Into ROI: Where Healthcare Leaders Should Start Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex
Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex