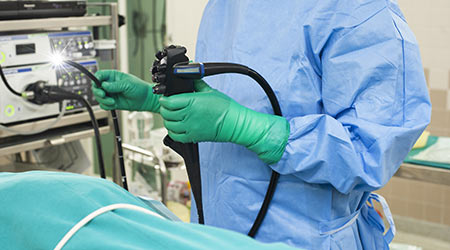
Preliminary results from the US Food and Drug Administration's (FDA's) mandated postmarket surveillance studies of duodenoscopes show "higher-than-expected" contamination rates after reprocessing, according to an article on the Medscape website.
The FDA said 3 percent of properly collected samples tested positive for more than 100 colony-forming units of "low-concern" organisms.
These organism are unlikely to cause serious infections but are, nevertheless, an indication of a "reprocessing failure."
An additional 3 percent of properly collected samples tested positive for "high-concern" bacteria that are more often associated with disease.
 Building Sustainable Healthcare for an Aging Population
Building Sustainable Healthcare for an Aging Population Froedtert ThedaCare Announces Opening of ThedaCare Medical Center-Oshkosh
Froedtert ThedaCare Announces Opening of ThedaCare Medical Center-Oshkosh Touchmark Acquires The Hacienda at Georgetown Senior Living Facility
Touchmark Acquires The Hacienda at Georgetown Senior Living Facility Contaminants Under Foot: A Closer Look at Patient Room Floors
Contaminants Under Foot: A Closer Look at Patient Room Floors Power Outages Largely Driven by Extreme Weather Events
Power Outages Largely Driven by Extreme Weather Events