The Food and Drug Administration is asking manufacturers to submit additional data about medical hand washes and sanitizers, including the long-term health effects of their daily use on the skin, according to an article on the ABC News website.
Under a proposed rule, companies must submit new studies looking at key safety issues, including possible hormonal effects and contributions to antibiotic-resistant bacteria.
Products that are not shown to be safe and effective by 2018 would have to be reformulated or removed from the market.
For now, the FDA said that healthcare workers should continue using hand washes, sanitizers and surgical scrubs, which are standard tools for preventing healthcare infections.

 Regulations Take the Lead in Healthcare Restroom Design
Regulations Take the Lead in Healthcare Restroom Design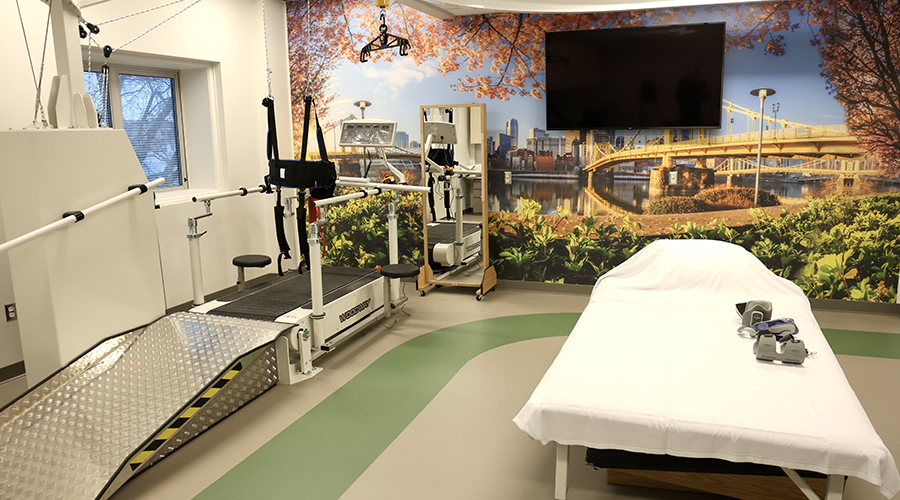 AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit
AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay
HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay Turning Facility Data Into ROI: Where Healthcare Leaders Should Start
Turning Facility Data Into ROI: Where Healthcare Leaders Should Start Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex
Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex