The U.S. Food and Drug Administration issued a proposed rule requesting additional scientific data to support the safety and effectiveness of certain active ingredients used in hand sanitizers.
The FDA’s request for more data is intended to help the agency ensure that regular use of these products does not present unknown safety and efficacy concerns, and does not mean the FDA believes these products are ineffective or unsafe.
Based on new scientific information, the agency is requesting additional scientific data to demonstrate that the active ingredients used in antiseptic rubs are generally recognized as safe and effective to reduce bacteria on skin.
The agency is requesting manufacturers provide data for three active ingredients — alcohol (ethanol or ethyl alcohol), isopropyl alcohol and benzalkonium chloride.

 From Downtime to Data: Rethinking Restroom Reliability in Healthcare
From Downtime to Data: Rethinking Restroom Reliability in Healthcare LeChase Building Four-Story Addition to UHS Delaware Valley Hospital
LeChase Building Four-Story Addition to UHS Delaware Valley Hospital AdventHealth Sebring Breaks Ground on Expansion Project
AdventHealth Sebring Breaks Ground on Expansion Project Regulations Take the Lead in Healthcare Restroom Design
Regulations Take the Lead in Healthcare Restroom Design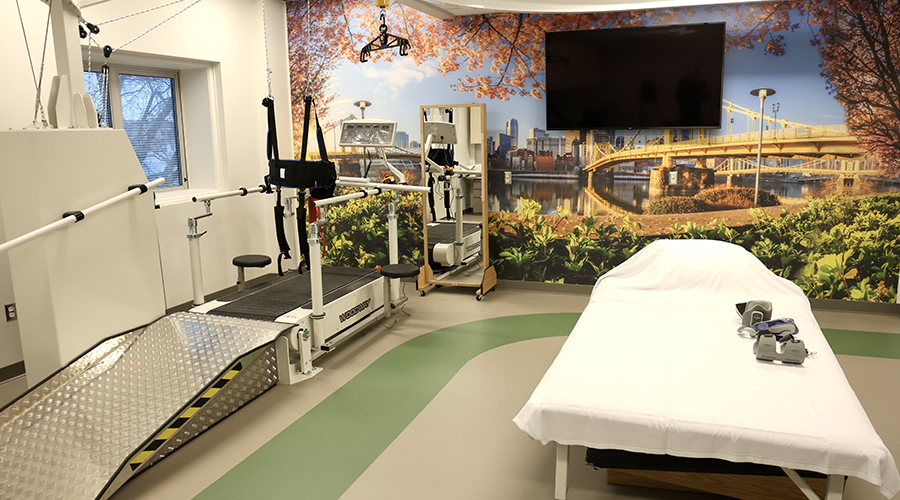 AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit
AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit