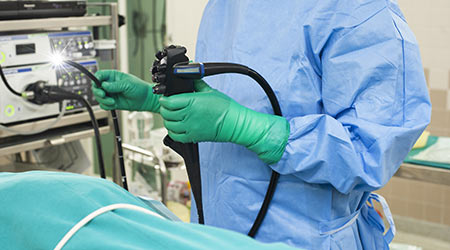
Preliminary results from the US Food and Drug Administration's (FDA's) mandated postmarket surveillance studies of duodenoscopes show "higher-than-expected" contamination rates after reprocessing, according to an article on the Medscape website.
The FDA said 3 percent of properly collected samples tested positive for more than 100 colony-forming units of "low-concern" organisms.
These organism are unlikely to cause serious infections but are, nevertheless, an indication of a "reprocessing failure."
An additional 3 percent of properly collected samples tested positive for "high-concern" bacteria that are more often associated with disease.
 What Lies Ahead for Healthcare Facilities Managers
What Lies Ahead for Healthcare Facilities Managers What's in the Future for Healthcare Restrooms?
What's in the Future for Healthcare Restrooms? Hammes Completes the Moffit Speros Outpatient Center
Hammes Completes the Moffit Speros Outpatient Center The Top Three Pathogens to Worry About in 2026
The Top Three Pathogens to Worry About in 2026 Blackbird Health Opens New Pediatric Mental Health Clinic in Virginia
Blackbird Health Opens New Pediatric Mental Health Clinic in Virginia