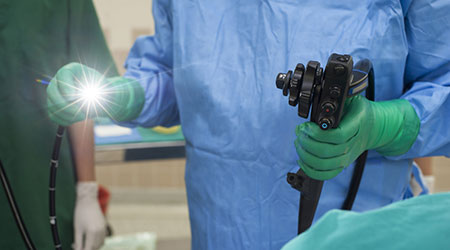
FDA recently granted Pentax approval to market a duodenoscope with fewer parts that need to be cleaned, according to an article on the Advisory Board website.
According to FDA, as many as 350 patients at 41 medical facilities across the globe were infected by or exposed to contaminated gastrointestinal scopes between 2010 and 2015.
Contaminated scopes had killed at least 35 patients in the United States from 2013 to January 2017.
FDA in 2013 started to collaborate with duodenoscope manufacturers Fujifilm, Olympus, and Pentax to address contamination risks.
 Mature Dry Surface Biofilm Presents a Problem for Candida Auris
Mature Dry Surface Biofilm Presents a Problem for Candida Auris Sutter Health's Arden Care Center Officially Opens
Sutter Health's Arden Care Center Officially Opens Insight Hospital and Medical Center Falls to Data Breach
Insight Hospital and Medical Center Falls to Data Breach The High Cost of Healthcare Violence
The High Cost of Healthcare Violence EVS Teams Can Improve Patient Experience in Emergency Departments
EVS Teams Can Improve Patient Experience in Emergency Departments