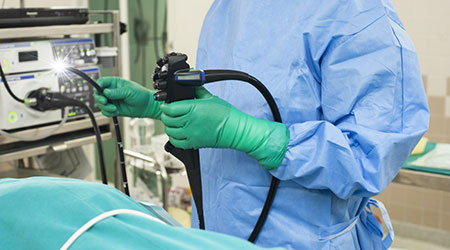
The Food and Drug Administration (FDA) said companies that make duodenoscopes should begin making disposable versions, according to an article on The New York Times website.
The agency also said that hospitals should start to transition to models with disposable components to reduce the risk of infection.
FDA tests revealed that one in 20 harbored disease-causing microbes like E. coli even after being properly cleaned.
Medical experts have urged the FDA to force manufacturers to make the scopes safer and easier to decontaminate — or to take them off the market.
 Assisted Living Facility Violated Safety Standards: OSHA
Assisted Living Facility Violated Safety Standards: OSHA McCarthy Completes Construction of Citizens Health Hospital in Kansas
McCarthy Completes Construction of Citizens Health Hospital in Kansas California Tower at UC Davis Health Topped Out
California Tower at UC Davis Health Topped Out What 'Light' Daily Cleaning of Patient Rooms Misses
What 'Light' Daily Cleaning of Patient Rooms Misses Sprinkler Compliance: Navigating Code Mandates, Renovation Triggers and Patient Safety
Sprinkler Compliance: Navigating Code Mandates, Renovation Triggers and Patient Safety