The Geisinger Health System of Danville, Pa., is transforming its supply chain into a care support services group by reorganizing and retraining staff to be more supportive of front-line care providers, according to an article on the Health Facilities Management website.
The Geisinger effort is one example of changes happening in hospitals around the country
More change is coming with the adoption of unique device identifiers (UDIs) for medical devices and associated technology to integrate medical device data into patient records and hospital data.
A new meaningful use rule by the Office of the National Coordinator on Health IT and the Centers for Medicare and Medicaid Services requires hospital electronic health record systems to include UDIs within the Common Clinical Data Sets.

 Regulations Take the Lead in Healthcare Restroom Design
Regulations Take the Lead in Healthcare Restroom Design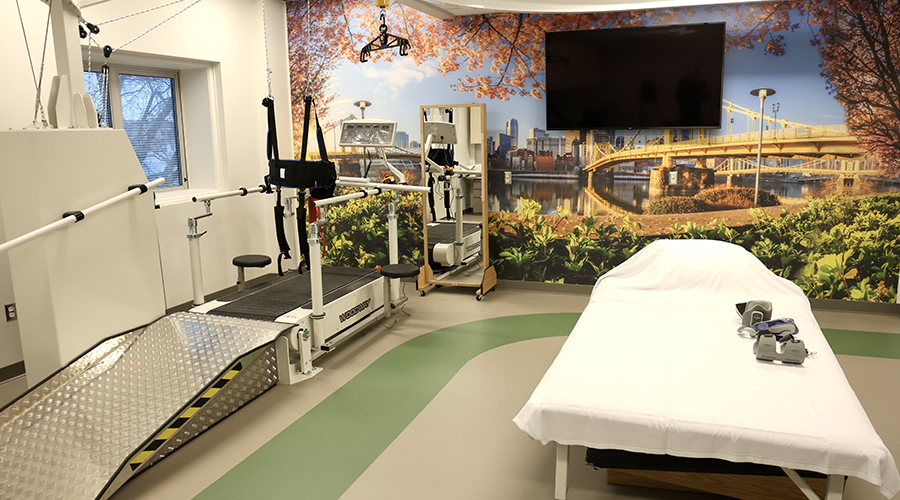 AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit
AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay
HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay Turning Facility Data Into ROI: Where Healthcare Leaders Should Start
Turning Facility Data Into ROI: Where Healthcare Leaders Should Start Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex
Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex