Effective July 1, 2014, hospitals must comply with two new environment of care elements of performance that align with new Centers for Medicare & Medicaid Services (CMS) guidelines from December 2013, according to an article on the Joint Commission website.
The guidelines clarify when a hospital may adjust its maintenance, inspection and testing activities for facility and medical equipment from what is recommended by the manufacturer.
To determine the best equipment maintenance strategies and frequencies, an organization should conduct a thorough risk assessment, which includes creating an equipment inventory. The organization must be able to describe the process used and have documentation to support its decision to deviate from the manufacturer’s recommendations to comply with CMS and Joint Commission requirements, the article said.
In 2013, CMS said a hospital may adjust its equipment maintenance, inspection, and testing frequency and activities from what is recommended by the manufacturer to a process that is based on a risk assessment by qualified personnel.
According to CMS, hospitals electing to use this alternative equipment management approach must develop policies and procedures and maintain documentation to support these activities.

 Regulations Take the Lead in Healthcare Restroom Design
Regulations Take the Lead in Healthcare Restroom Design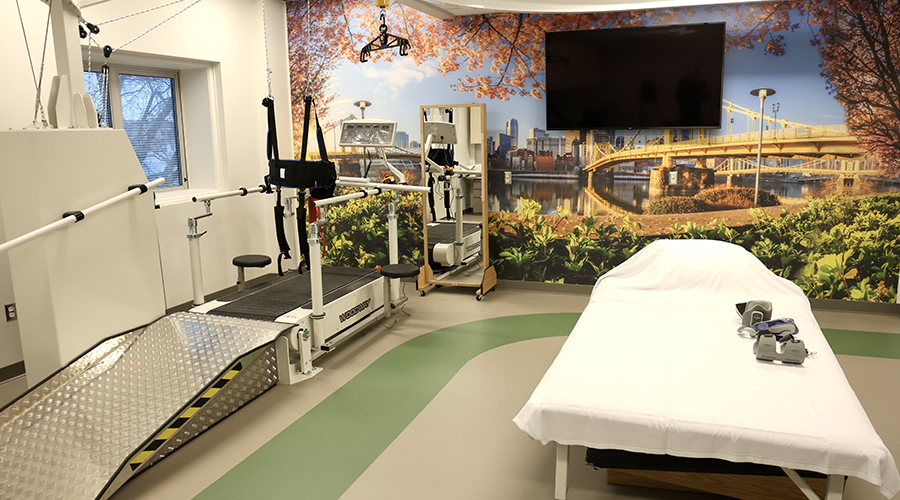 AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit
AHN Allegheny Valley Hospital Opens Expanded Inpatient Rehabilitation Unit HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay
HSHS and Lifepoint Rehabilitation Partner on New Inpatient Rehab Hospital in Green Bay Turning Facility Data Into ROI: Where Healthcare Leaders Should Start
Turning Facility Data Into ROI: Where Healthcare Leaders Should Start Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex
Sutter Health Breaks Ground on Advanced Cancer Center and Care Complex